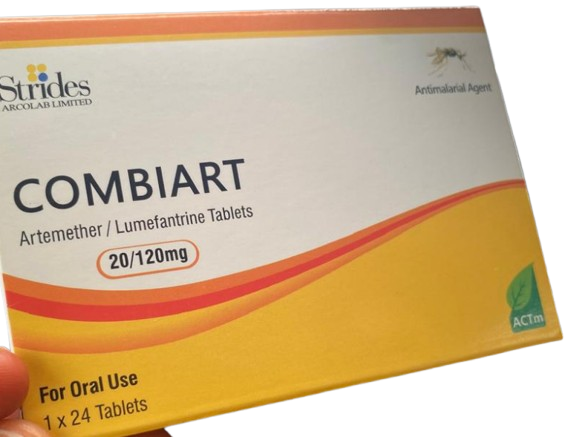
The National Agency for Food and Drug Administration and Control (NAFDAC) has sounded the alarm over the circulation of counterfeit Combiart Dispersible Tablet 20/120mg in Nigeria. In a statement shared on its X (formerly Twitter) handle on Thursday, the agency disclosed that the fake product, manufactured by Strides Arcolab Limited in India, was discovered in the Federal Capital Territory (FCT) and Rivers State during surveillance activities.
NAFDAC revealed that laboratory tests on the counterfeit product showed it contained no active pharmaceutical ingredients, rendering it ineffective. The product also displayed discrepancies in date markings, with the NAFDAC registration number found to be invalid and unrelated to the product. Additionally, the license for the original product has expired, according to the agency’s database.
The counterfeit medicine contains Artemether and Lumefantrine, commonly used to treat uncomplicated malaria caused by mosquito bites. However, NAFDAC clarified that it is not intended for severe or complicated malaria cases. The agency warned that counterfeit medicines pose severe health risks due to their inability to meet regulatory standards, resulting in compromised safety, quality, and efficacy. These medicines can lead to adverse health outcomes, including death, as they fail to effectively treat conditions.
The agency has directed its officials to intensify surveillance and remove the counterfeit products from circulation. It urged stakeholders across the pharmaceutical supply chain, including importers, distributors, and retailers, to remain vigilant and ensure that medicines are sourced only from authorized suppliers. NAFDAC emphasized the importance of verifying the authenticity and physical condition of all medical products.
Healthcare professionals and the public are encouraged to report any suspicion of substandard or falsified medicines to NAFDAC through its various reporting channels, including a toll-free hotline, email, and its e-reporting platform. Reports of adverse effects or side effects can also be submitted via the Med-Safety app or email.
NAFDAC’s actions underline its commitment to safeguarding public health by ensuring that only quality medicines are available to Nigerians.NAFDAC Alerts Nigerians to Counterfeit Combiart Tablets